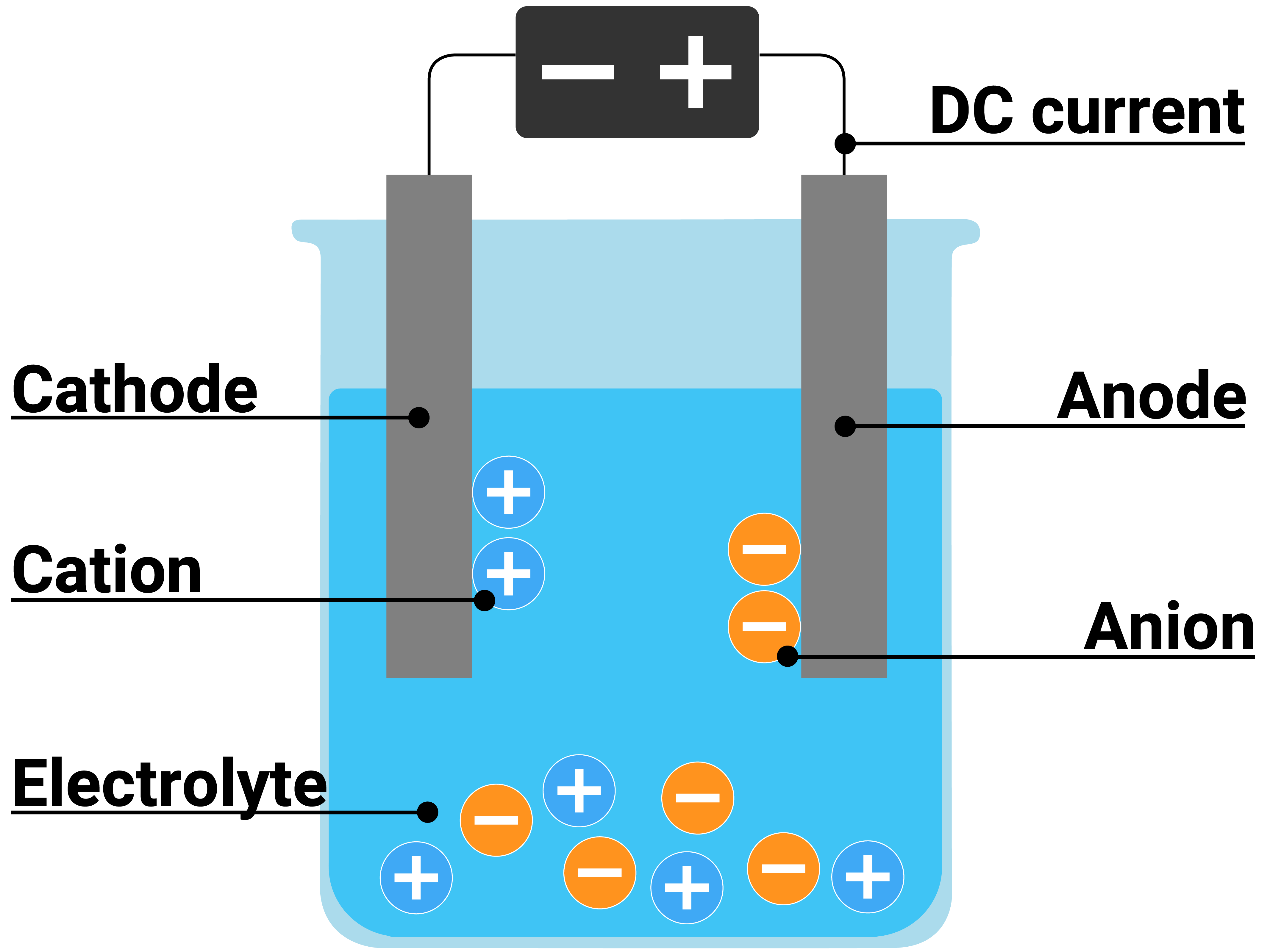
Magnesium Chloride Electrolysis Equation . electrolysis involves using electricity to break down electrolytes to form elements. solve your electrochemical problems with our faraday's law of electrolysis calculator! to illustrate the essential concepts of electrolysis, a few specific processes will be considered. The products of electrolysis can be. electrolysis is done in solutions, which contain enough ions so current can flow. electrolysis separates the molten ionic compound into its elements. If the solution contains only one material, like the. Electrochemistry is the perfect fusion of physics. according to the equations, three moles of electrons produce one mole of iron and 2 moles of electrons produce 1 mole of. magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes.
from quizizz.com
electrolysis is done in solutions, which contain enough ions so current can flow. according to the equations, three moles of electrons produce one mole of iron and 2 moles of electrons produce 1 mole of. magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. If the solution contains only one material, like the. electrolysis separates the molten ionic compound into its elements. electrolysis involves using electricity to break down electrolytes to form elements. solve your electrochemical problems with our faraday's law of electrolysis calculator! to illustrate the essential concepts of electrolysis, a few specific processes will be considered. The products of electrolysis can be. Electrochemistry is the perfect fusion of physics.
Electrolysis of Aqueous Solutions 221 plays Quizizz
Magnesium Chloride Electrolysis Equation solve your electrochemical problems with our faraday's law of electrolysis calculator! electrolysis is done in solutions, which contain enough ions so current can flow. Electrochemistry is the perfect fusion of physics. magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. electrolysis involves using electricity to break down electrolytes to form elements. according to the equations, three moles of electrons produce one mole of iron and 2 moles of electrons produce 1 mole of. If the solution contains only one material, like the. The products of electrolysis can be. to illustrate the essential concepts of electrolysis, a few specific processes will be considered. electrolysis separates the molten ionic compound into its elements. solve your electrochemical problems with our faraday's law of electrolysis calculator!
From www.nagwa.com
Question Video Identifying the Equation That Describes What Happens to Magnesium Chloride Electrolysis Equation electrolysis separates the molten ionic compound into its elements. solve your electrochemical problems with our faraday's law of electrolysis calculator! magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. to illustrate the essential concepts of electrolysis, a few specific processes will be considered. If the solution contains only one material, like. Magnesium Chloride Electrolysis Equation.
From www.melford.co.uk
M24000100.0 Magnesium Chloride Hexahydrate, 100 Grams Magnesium Chloride Electrolysis Equation electrolysis involves using electricity to break down electrolytes to form elements. magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. If the solution contains only one material, like the. solve your electrochemical problems with our faraday's law of electrolysis calculator! electrolysis separates the molten ionic compound into its elements. to. Magnesium Chloride Electrolysis Equation.
From www.thesciencehive.co.uk
Mass and Mole Calculations (AQA) — the science hive Magnesium Chloride Electrolysis Equation according to the equations, three moles of electrons produce one mole of iron and 2 moles of electrons produce 1 mole of. solve your electrochemical problems with our faraday's law of electrolysis calculator! Electrochemistry is the perfect fusion of physics. electrolysis involves using electricity to break down electrolytes to form elements. electrolysis separates the molten ionic. Magnesium Chloride Electrolysis Equation.
From saylordotorg.github.io
Electrochemistry Magnesium Chloride Electrolysis Equation magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. If the solution contains only one material, like the. according to the equations, three moles of electrons produce one mole of iron and 2 moles of electrons produce 1 mole of. electrolysis involves using electricity to break down electrolytes to form elements. . Magnesium Chloride Electrolysis Equation.
From www.cbsetuts.com
What is the chemical formula of magnesium chloride? CBSE Tuts Magnesium Chloride Electrolysis Equation according to the equations, three moles of electrons produce one mole of iron and 2 moles of electrons produce 1 mole of. electrolysis is done in solutions, which contain enough ions so current can flow. The products of electrolysis can be. If the solution contains only one material, like the. Electrochemistry is the perfect fusion of physics. . Magnesium Chloride Electrolysis Equation.
From www.tessshebaylo.com
Electrolysis Of Salt Water Half Equations Tessshebaylo Magnesium Chloride Electrolysis Equation The products of electrolysis can be. Electrochemistry is the perfect fusion of physics. If the solution contains only one material, like the. electrolysis separates the molten ionic compound into its elements. according to the equations, three moles of electrons produce one mole of iron and 2 moles of electrons produce 1 mole of. electrolysis is done in. Magnesium Chloride Electrolysis Equation.
From www.toppr.com
Draw electron dot representation for the formation of magnesium chloride. Magnesium Chloride Electrolysis Equation according to the equations, three moles of electrons produce one mole of iron and 2 moles of electrons produce 1 mole of. electrolysis is done in solutions, which contain enough ions so current can flow. electrolysis involves using electricity to break down electrolytes to form elements. to illustrate the essential concepts of electrolysis, a few specific. Magnesium Chloride Electrolysis Equation.
From www.youtube.com
3.26 electrolysis of magnesium chloride YouTube Magnesium Chloride Electrolysis Equation to illustrate the essential concepts of electrolysis, a few specific processes will be considered. according to the equations, three moles of electrons produce one mole of iron and 2 moles of electrons produce 1 mole of. solve your electrochemical problems with our faraday's law of electrolysis calculator! magnesium metal is produced by the electrolysis of molten. Magnesium Chloride Electrolysis Equation.
From www.chemicalslearning.com
What is the Reaction of Magnesium Chloride and Sodium Hydroxide? Magnesium Chloride Electrolysis Equation electrolysis is done in solutions, which contain enough ions so current can flow. The products of electrolysis can be. Electrochemistry is the perfect fusion of physics. If the solution contains only one material, like the. magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. according to the equations, three moles of electrons. Magnesium Chloride Electrolysis Equation.
From www.numerade.com
Magnesium metal is produced by the electrolysis of molten magnesium Magnesium Chloride Electrolysis Equation to illustrate the essential concepts of electrolysis, a few specific processes will be considered. electrolysis separates the molten ionic compound into its elements. according to the equations, three moles of electrons produce one mole of iron and 2 moles of electrons produce 1 mole of. electrolysis involves using electricity to break down electrolytes to form elements.. Magnesium Chloride Electrolysis Equation.
From signalticket9.pythonanywhere.com
Unbelievable Magnesium Chloride Balanced Equation Maths Formulas For Magnesium Chloride Electrolysis Equation electrolysis involves using electricity to break down electrolytes to form elements. Electrochemistry is the perfect fusion of physics. according to the equations, three moles of electrons produce one mole of iron and 2 moles of electrons produce 1 mole of. magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. solve your. Magnesium Chloride Electrolysis Equation.
From www.numerade.com
SOLVED Magnesium metal reacts with chlorine gas, Cl2, to produce Magnesium Chloride Electrolysis Equation to illustrate the essential concepts of electrolysis, a few specific processes will be considered. electrolysis is done in solutions, which contain enough ions so current can flow. magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. electrolysis separates the molten ionic compound into its elements. according to the equations, three. Magnesium Chloride Electrolysis Equation.
From www.researchgate.net
Cell for hydrous magnesium chloride electrolysis (schematic) Download Magnesium Chloride Electrolysis Equation The products of electrolysis can be. to illustrate the essential concepts of electrolysis, a few specific processes will be considered. electrolysis is done in solutions, which contain enough ions so current can flow. If the solution contains only one material, like the. according to the equations, three moles of electrons produce one mole of iron and 2. Magnesium Chloride Electrolysis Equation.
From www.numerade.com
SOLVED Solid silicon and solid magnesium chloride form when silicon Magnesium Chloride Electrolysis Equation Electrochemistry is the perfect fusion of physics. electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be. solve your electrochemical problems with our faraday's law of electrolysis calculator! electrolysis separates the molten ionic compound into its elements. magnesium metal is produced by the electrolysis of molten magnesium chloride using. Magnesium Chloride Electrolysis Equation.
From quizizz.com
Electrolysis of Aqueous Solutions 221 plays Quizizz Magnesium Chloride Electrolysis Equation electrolysis involves using electricity to break down electrolytes to form elements. solve your electrochemical problems with our faraday's law of electrolysis calculator! to illustrate the essential concepts of electrolysis, a few specific processes will be considered. electrolysis is done in solutions, which contain enough ions so current can flow. Electrochemistry is the perfect fusion of physics.. Magnesium Chloride Electrolysis Equation.
From www.i-ciencias.com
[Resuelta] físicoquímica Ánodo/Cátodo positivo o Magnesium Chloride Electrolysis Equation If the solution contains only one material, like the. electrolysis separates the molten ionic compound into its elements. electrolysis is done in solutions, which contain enough ions so current can flow. electrolysis involves using electricity to break down electrolytes to form elements. solve your electrochemical problems with our faraday's law of electrolysis calculator! magnesium metal. Magnesium Chloride Electrolysis Equation.
From www.toppr.com
How is magnesium chloride formed by the transfer of electrons? Why does Magnesium Chloride Electrolysis Equation electrolysis involves using electricity to break down electrolytes to form elements. according to the equations, three moles of electrons produce one mole of iron and 2 moles of electrons produce 1 mole of. solve your electrochemical problems with our faraday's law of electrolysis calculator! The products of electrolysis can be. If the solution contains only one material,. Magnesium Chloride Electrolysis Equation.
From selfdirectedce.com
How to Write the Net Ionic Equation for Mg + CuCl2 = MgCl2 + Cu Magnesium Chloride Electrolysis Equation Electrochemistry is the perfect fusion of physics. to illustrate the essential concepts of electrolysis, a few specific processes will be considered. solve your electrochemical problems with our faraday's law of electrolysis calculator! electrolysis separates the molten ionic compound into its elements. If the solution contains only one material, like the. according to the equations, three moles. Magnesium Chloride Electrolysis Equation.